BIO-RISE CHINA: A Randomized Trial of a Biolimus-Coated Balloon Versus Plain Old Balloon Angioplasty in Small Vessel Coronary Artery Disease
ClinicalTrials.gov Identifier: NCT03769623
Brief Summary:
This study is a prospective, multicenter, randomized, blind, parallel and superiority test study. It is planned to select 206 cases of subjects with small coronary artery vessel disease who meet the inclusion/exclusion criteria. They are randomly divided into Biolimus A9™ (BA9™) release coronary balloon catheter treatment group and plain old balloon angioplasty catheter (Powerline™) treatment group according to the ratio of 1:1. All subjects accept clinical follow-up after operation, at 30 days, 6 months, 9 months and 12 months after operation. Follow-up with angiography is conducted at 9 months.
Primary end-point:
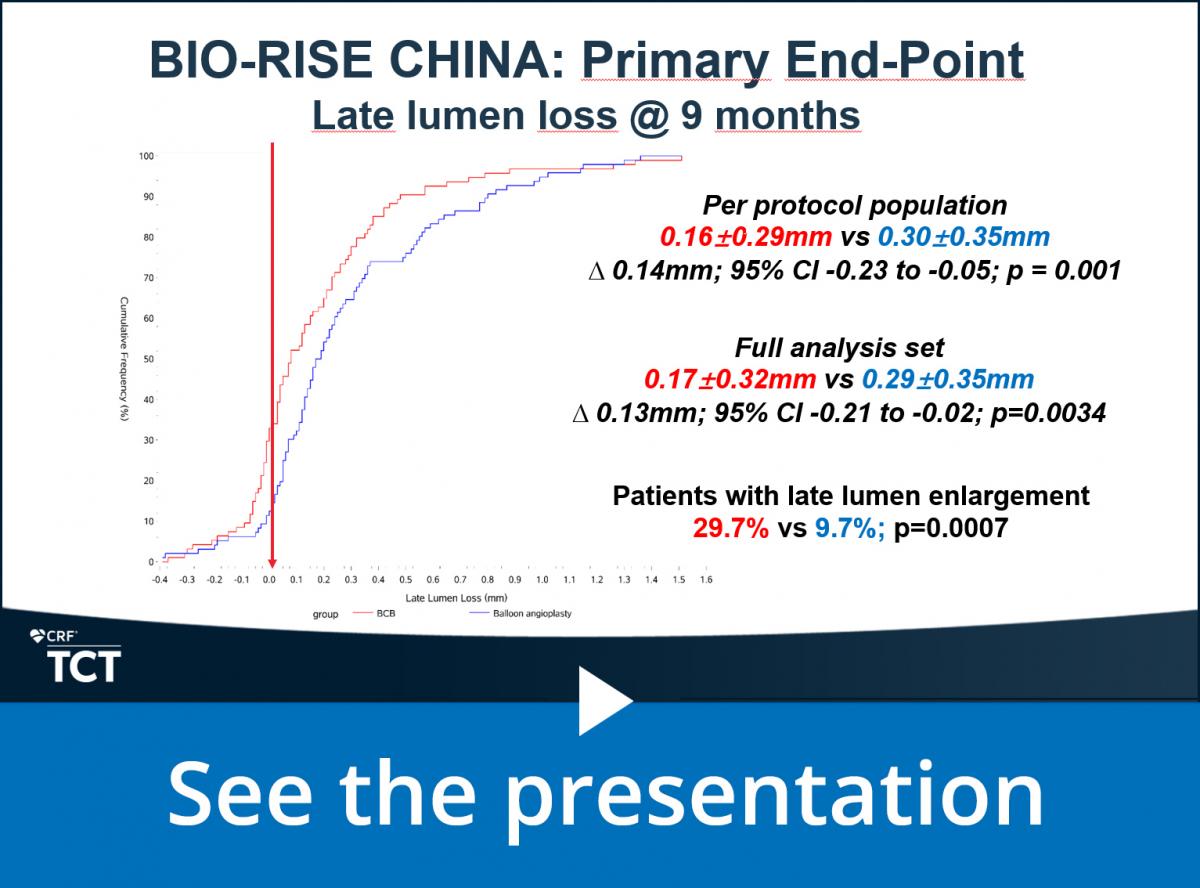
In-segment late lumen loss (LLL) at 9 months assessed by an independent core-lab blinded to treatment assignment.
Secondary end-points:
- Device and procedural success
- Binary restenosis
- Target lesion failure (TLF) at 30 days, 6, 9 and 12 months
- Patient orientated clinical outcome (POCO) defined as a composite of all-cause death, myocardial infarction and any revascularization at the same time points
Study device:
The Biolimus A9™ DCB is an investigational device and is not yet approved for sales. It features the following caracteristics:
- Balloon: semi-compliant angioplasty balloon
- Coating: 3 µg/mm2 Crystalline Biolimus A9. BA9™ is a highly lipophilic anti-restenotic drug and was specifically developed by Biosensors International for local drug delivery into vascular tissue.
- Excipient: polyethylene oxide (PEO)
- Mechanism of action: Ultra fast direct transfer of the highly lipophilic1 BA9™ to vessel wall on balloon expansion.
Results:
In a Late Breaking Clinical Trials session at TCT2021, Dr. Yaling Han presented the following results:
0.16±0.29mm vs 0.30±0.35mm ∆ 0.14mm; 95% CI -0.23 to -0.05; p=0.001 (per protocol population) and concluded:
- “This first-in-human randomized controlled trial has confirmed the safety and efficacy of a biolimus coated balloon in patients with small vessel coronary artery disease compared to plain old balloon angioplasty;
- Late lumen enlargement was 3 times more common with the biolimus coated balloon;
- There was a trend towards improved clinical outcomes at 1 year;
- Future studies are warranted to compare the BCB to DES and/or other DCBs”.
1. Biosensors International data on file.
Sources:
https://www.clinicaltrials.gov.
BIO-RISE CHINA: A Randomized Trial of a Biolimus-Coated Balloon Versus Plain Old Balloon Angioplasty in Small Vessel Coronary Artery Disease. PI: Dr. Yaling Han, Site PI: Dr. Kai Xu, On behalf of the BIO-RISE CHINA Investigators