CLINICAL EVIDENCE OF HEMODYNAMIC PERFORMANCE
The EMPIRE I study6 data confirms the superior hemodynamic performance of ALLEGRA™.
PREVENTION OF PATIENT PROSTHESIS MISMATCH (PPM)
ALLEGRA™ showed no severe or moderate PPM in native valve anatomies in the EMPIRE I study.6
Patient Prosthesis Mismatch (PPM) is a predictor of mortality and must be prevented for patient long-term survival.7 The ALLEGRA™ supports the prevention of PPM.6
COMPARISON OF PPM RATES IN NATIVE VALVES
ALLEGRA™ has shown no severe or moderate PPM in comparison to all other contemporary valve platforms.

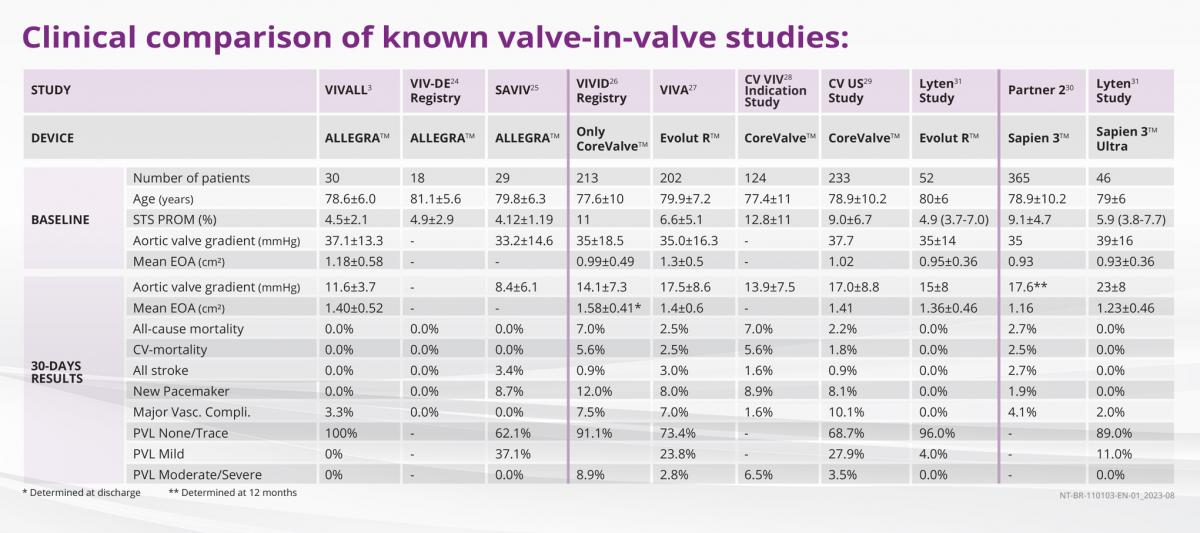
THE EXTRAORDINARY OUTCOMES IN VALVE-IN-VALVE PROCEDURES
Clinical data demonstrate the excellent suitability of ALLEGRA™ in Valve-in-Valve applications.
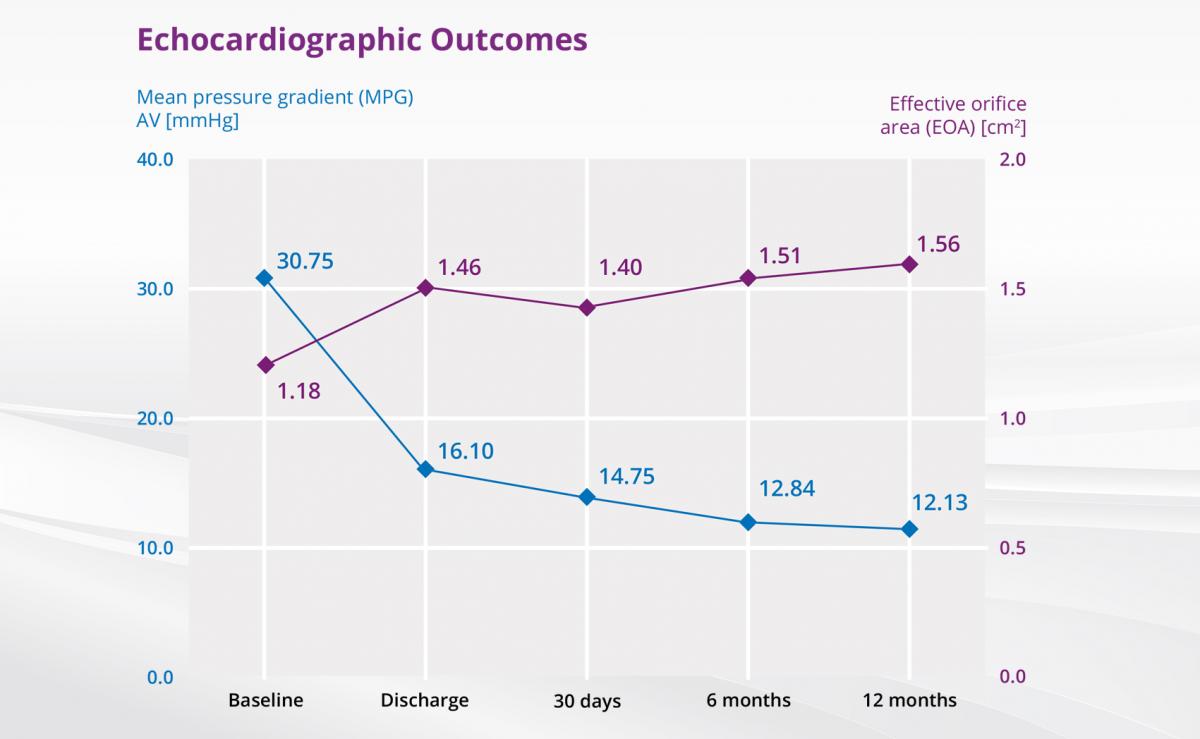
The VIVALL study3 investigated the safety and performance of the ALLEGRA™ in patients with failed surgical aortic bioprosthetic valves.
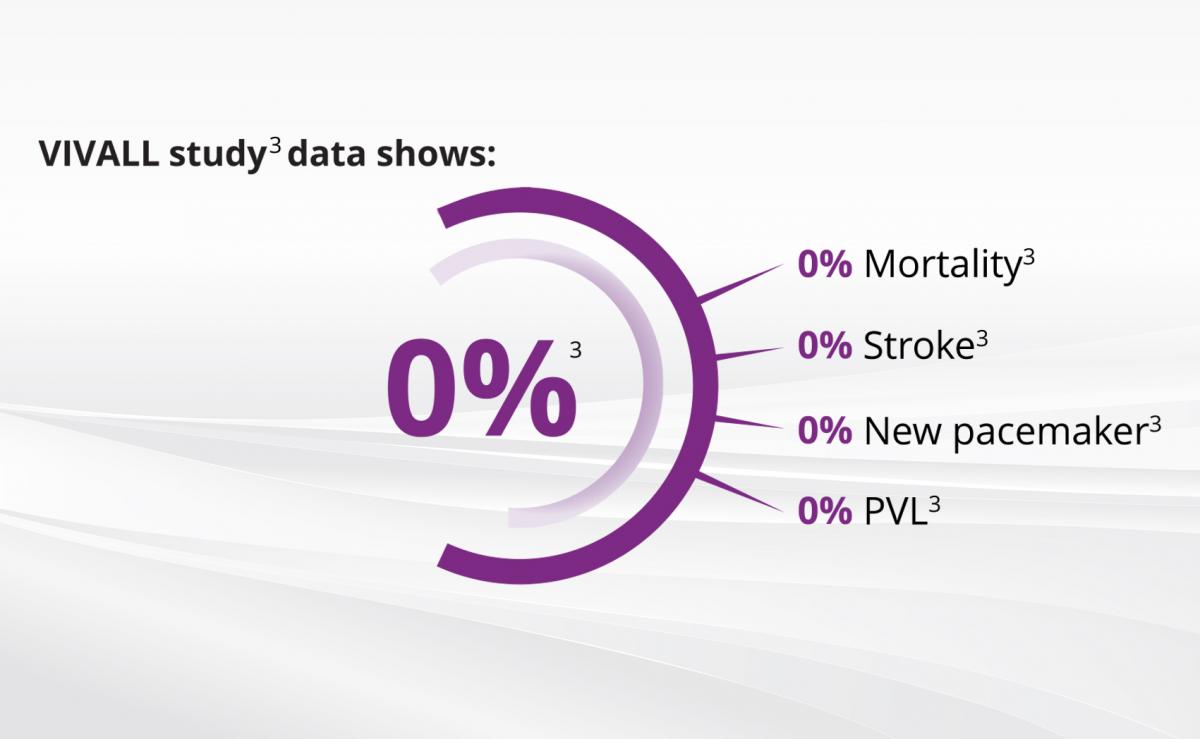
EXCELLENT SUITABILITY IN VALVE-IN-VALVE
The VIVALL study investigated the safety and performance of the ALLEGRA™ in Valve-in-Valve and showed best clinical results:
- 0% PVL, 0% new pacemakers, 0% stroke, and 0% mortality.
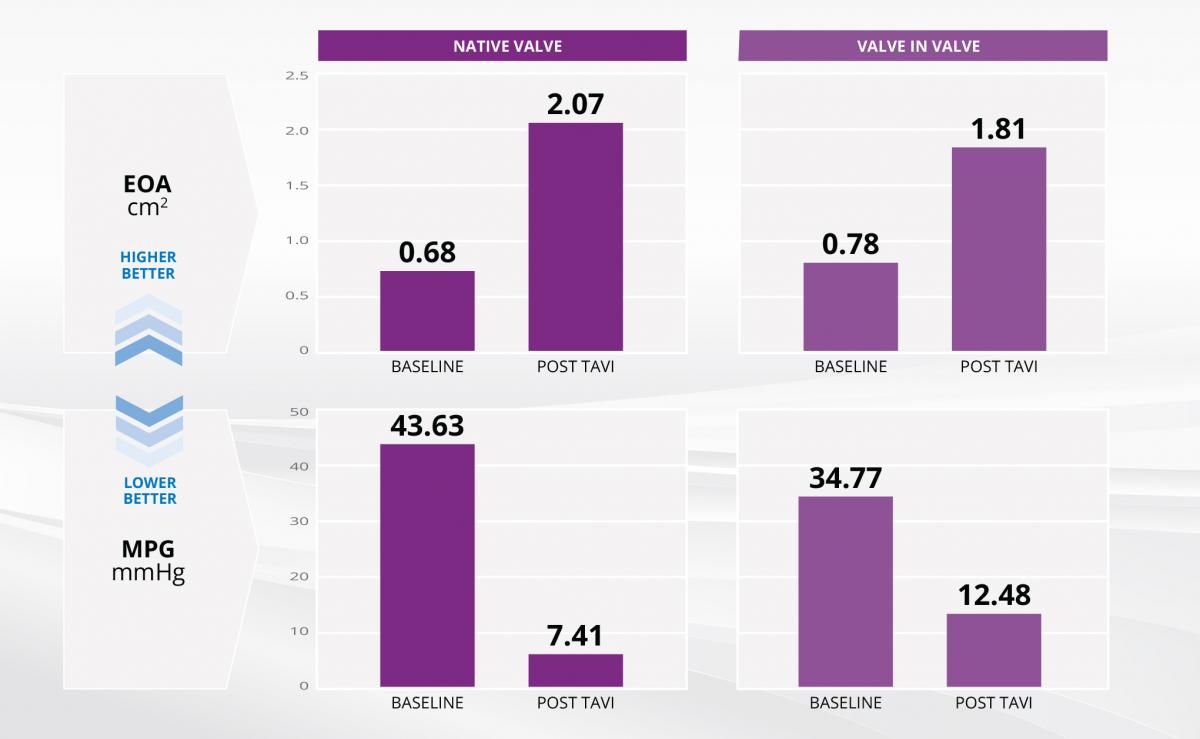
- "Best-in-class" hemodynamics
- Lowest pressure gradients and highest effective orifice areas compared with other studies
- 80% of the surgical bioprostheses had a true inner diameter of ≤21 mm
COMPARISON OF VALVE-IN-VALVE STUDIES
ALLEGRA™ also shows excellent results in direct comparison to other Valve-in-Valve studies:
- Lowest pressure gradients in the study comparison shown
- No moderate or severe PVL at 30 days
- Very good safety profile (mortality, stroke or vascular complications)