UNCOMPROMISED HEMODYNAMICS. BY DESIGN.
The ALLEGRA™ Transcatheter Heart Valve (THV) has been designed for uncompromised hemodynamic performance with single digit mean pressure gradients and high effective orifice areas.1-3
The ALLEGRA™ THV is indicated for the treatment of severe calcified aortic valve stenosis in high risk patients with elevated surgical risk or in patients with a symptomatic degeneration of an aortic valve bioprosthesis.

UNIQUE STENT FRAME
The ALLEGRA™ maximizes the effective orifice area.
The self-expanding unique stent frame of ALLEGRA™ has convex and concave area for high effective orifice area.
ANCHORING AND SEALING
The ALLEGRA™ stent frame has tailored radial force for effective anchoring and sealing in the annulus.
The stent frame design provides high radial force in the sealing area and less radial force at the outflow.
WIDER VALVE OPENING
The ALLEGRA™ is a supra-annular valve with a flexible stent outflow for wider valve opening.
The flexible stent outflow allows for wider valve opening for large EOA and low mean pressure gradient.1-3
SMALL ANNULI AND VALVE-IN-VALVE
ALLEGRA™ maximizes the hemodynamic performance in small native annuli and in Valve-in-Valve procedures.4-5
With a wide valve opening and supra-annular valve position, the patient can benefit from high EOA and low mean pressure gradients.1-3 Minimum treatable annulus diameter is 16.5 mm.

DURABILITY
The ALLEGRA™ stent frame allows for flexing at the commissural fixation points.
Flexible commissural fixation points reduce stress to the leaflets in every cardiac cycle and are associated with increased durability.20,21
TISSUE PROTECTION
The ALLEGRA™ is protected by TissGUARD™ for long-term valve function.
TissGUARD™ technology combines the bovine pericardial tissue anti-calcification treatment with leaflet stress-reduction effects of flexible commissural fixation points resulting in long-term valve function.
CORONARY ACCESS
ALLEGRA™ maintains access to the coronary ostia for future interventions.
The 6 radiopaque gold markers highlight where the top of the sealing skirt is and ensures precise implantation for future coronary access.
FLOW DYNAMICS
ALLEGRA™ shows in-vitro a more physiological flow pattern than other valves.
Achieving a physiologic flow pattern results in lower gradients, less platelet activation, hemolysis and minimal paravalvular leak resulting in a longer lasting valve.23

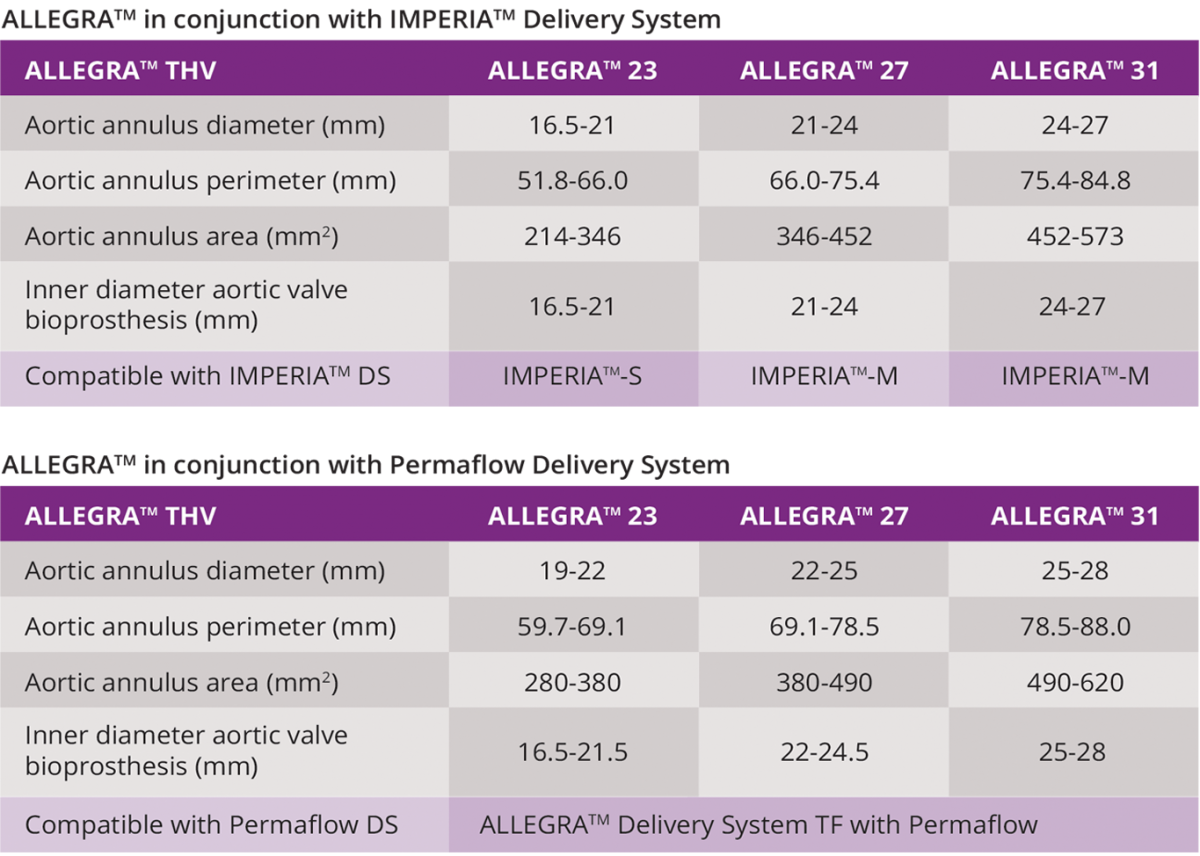
ALLEGRATM THV SPECIFICATIONS